[Courtesy of UNIST]
Normally, hydrogen fuel is stored in a pressurized, low-temperature liquid state or as ammonia to be broken down into hydrogen atoms at fueling stations. Such storage techniques require high energy costs. Pressurized hydrogen also poses a safety threat. Because of safety issues, South Koreans have been locked in a debate on how to establish urban hydrogen vehicle fuel stations in populated areas.
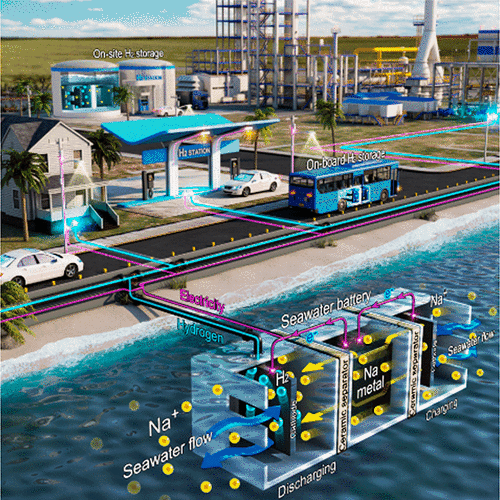
A seawater battery uses lithium to oxidize and deoxidize sodium-ion to store and discharge electricity. Unlike ordinary secondary batteries, seawater batteries are based on an open-structured cathode compartment that allows gaseous dioxygen (O2) from the ambient air to enter seawater to cause chemical reactions. Electric power and hydrogen gas are produced in the process.
The Ulsan National Institute of Science and Technology (UNIST) said that its research team has developed an underwater hydrogen energy storage system (ESS) based on a seawater battery. The system uses alkali-metal-based storage made with relatively light alkali metals such as lithium and sodium.
"We have developed a novel hydrogen storage technique capable of producing hydrogen and charging electricity simultaneously using a battery system that uses seawater, an infinite energy source," UNIST head researcher Jang Ji-wook was quoted as saying.
Researchers found that the new alkali-metal-based hydrogen storage showed 99.1 percent in faraday efficiency in the ordinary working environment of seawater batteries. The test-lab seawater battery with a size of 70 square centimeters sowed a faraday efficiency of 94.7 percent. Faraday efficiency refers to the efficiency with which charge is transferred in a system that facilitates an electrochemical reaction.
Copyright ⓒ Aju Press All rights reserved.




