SEOUL, March 06 (AJP) - A high-stakes competition in the weight-loss drug market is set to unfold in South Korea this year as Eli Lilly prepares to launch its GLP-1 medication, Mounjaro, a treatment that has demonstrated stronger weight-reduction effects than Novo Nordisk’s Wegovy, which debuted in the country late last year.
According to medical sources, Eli Lilly is poised to introduce Mounjaro in its “single-dose vial” form as early as the first half of this year.
The drug, a once-weekly injectable treatment for obesity and diabetes, has already secured regulatory approval in South Korea for its “prefilled pen” version — receiving authorization as a diabetes treatment in June 2023 and as an obesity treatment in August 2024. However, due to supply constraints, the prefilled pen is not expected to be available until November 2025.
In an effort to expedite its market entry, Eli Lilly plans to first roll out the single-dose vial and the “QuickPen” version, which contains a month’s supply — four doses — in a single pen.
“We applied for marketing approval for the single-dose vial and QuickPen at the end of last year,” an Eli Lilly representative said. “Launch timing will depend on the approval status of each product.”
Since Novo Nordisk introduced its earlier GLP-1 weight-loss drug, Saxenda, to the South Korean market in 2018, the country’s prescription weight-loss drug sector has nearly doubled in value, growing from 96.8 billion won to 178 billion won in 2023.
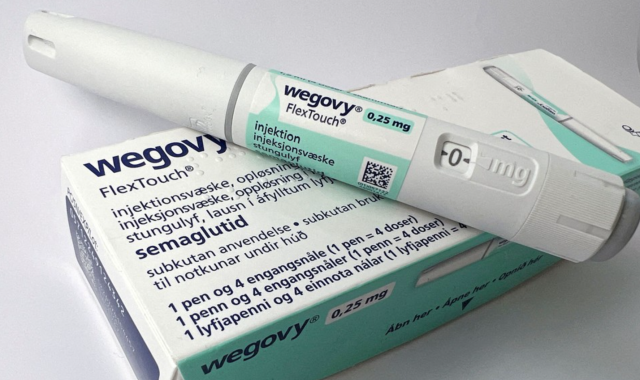
Novo Nordisk followed up by launching Wegovy in October 2024, touting superior efficacy and convenience over Saxenda. While Saxenda demonstrated an average weight reduction of 7.5 percent over 56 weeks in clinical trials, Wegovy showed a 14.9 percent reduction over 68 weeks.
Mounjaro, however, has delivered even more striking results, achieving a 22.5 percent weight reduction in 72-week clinical trials and 26.6 percent in 84-week trials — making it the only drug to surpass the highly sought-after 20 percent threshold. This reputation has driven some patients to seek the drug through illegal imports from countries such as Japan while awaiting its official release in South Korea.
Pricing remains a key factor in the competition. In South Korea, the monthly wholesale price of Wegovy is set at 372,025 won, with patients typically paying between 400,000 and 600,000 won.
While Eli Lilly has yet to disclose Mounjaro’s price in South Korea, its highest-dosage prefilled pen (15mg) costs roughly 450,000 won per month in Japan and approximately 1.5 million won in the United States.
However, the introduction of the vial version in the U.S. led to a price drop to about 730,000 won. Industry observers expect the Korean vial version to be priced lower than Japan’s prefilled pen and potentially undercut Wegovy’s price.
Eli Lilly is also aiming to secure national health insurance coverage for Mounjaro as a diabetes treatment, recently submitting an application to South Korea’s Health Insurance Review and Assessment Service.
“If a drug first enters the relatively high-priced, non-covered market, obtaining subsequent insurance coverage becomes more difficult,” said Park Tae-sun, a professor of endocrinology and metabolism at Jeonbuk National University Hospital. “For low-income patients with diabetes and obesity, it is crucial to include the treatment in health insurance coverage as soon as possible.”
Copyright ⓒ Aju Press All rights reserved.