LG Chem said Feb. 20 that an independent data monitoring committee (IDMC) recommended continuing the Phase 3 clinical trial of ficlatuzumab, a head and neck cancer drug being developed by its U.S. subsidiary, AVEO.
Based on interim results, the IDMC recommended selecting the higher of two tested doses — 20 mg/kg — as the final dose and proceeding with the study.
Ficlatuzumab is a monoclonal antibody targeted cancer therapy designed to inhibit the action of hepatocyte growth factor (HGF), which is involved in tumor growth and metastasis. The trial enrolls patients with HPV-negative head and neck cancer who previously received platinum-based chemotherapy and immune checkpoint inhibitors either sequentially as monotherapy or in combination. It compares ficlatuzumab plus cetuximab with placebo plus cetuximab.
LG Chem plans to recruit 410 to 500 patients and assess overall survival (OS), defined as the time from treatment start to death.

GC Biopharma said Feb. 20 it will run a relay webinar series from Feb. 25 through April 29 on its drug information site, GC Connect, under the theme “Practical clinical solutions and the latest insights on five key endocrine diseases.”
The company will invite 10 endocrinologists in private practice to deliver lectures tailored to primary care settings. The first session will cover the latest diabetes updates based on the 2025 Korean Diabetes Association (KDA) clinical practice guidelines and the American Diabetes Association (ADA) guidance. Subsequent webinars will address thyroid disease management, obesity drug treatment strategies, bone metabolism (osteoporosis) and metabolic syndrome (CKM syndrome), summarizing academic information on five major endocrine conditions.
GC Biopharma said the series is designed to offer practical clinical approaches to real-world challenges clinicians face in patient care by featuring speakers familiar with day-to-day practice settings.

Cho-A Pharma said Feb. 20 it launched Hepatos Syrup, packaged with its patented Cho-A Sepiji ampoule container.
Hepatos Syrup is an over-the-counter medicine that the company said helps protect liver cells and improve liver function through the combined effects of three ingredients: arginine, betaine and citric acid. As a liquid, it is designed for rapid absorption and quick effects related to liver energy synthesis and detoxification, the company said.
The main ingredient, arginine, is a precursor of urea and is essential for neutralizing toxic ammonia. Betaine, an oxidized derivative of choline, lowers cholesterol and increases bile secretion during choline metabolism, supporting fat metabolism and digestion, the company said. Citric acid is involved in amino acid and carbohydrate and lipid metabolism and promotes energy (ATP) production, which the company said can help relieve fatigue and improve endurance.
Cho-A said the Sepiji ampoule uses a material certified by the U.S. Pharmacopeia as the highest safety grade, USP Plastic Class VI. The company said it has registered patents in 30 countries including South Korea, the United States, Europe, Japan and Vietnam, and received certification from the nationally accredited testing institution KOTITI that no endocrine-disrupting chemicals were detected.
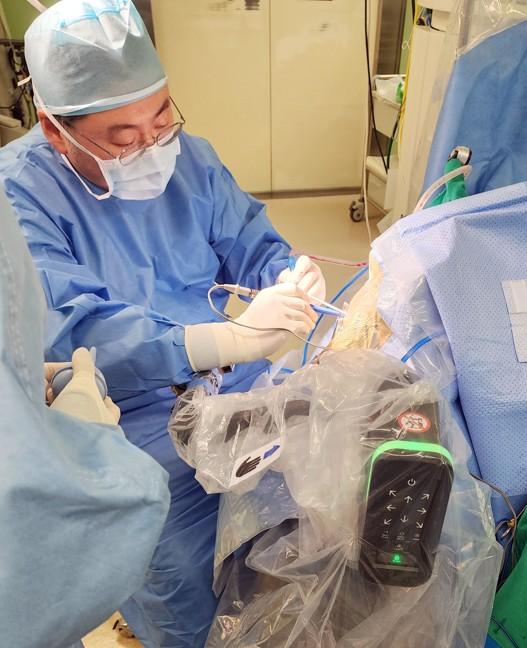
Severance Hospital said Feb. 20 it recently reached 500 cases of robot-assisted precision brain surgery, 5 years and 3 months after its first procedure in 2020.
The technique is used for precision procedures such as inserting electrodes into specific brain locations, collecting tissue samples and placing catheters. After a target is set on preoperative imaging, a robotic arm automatically guides the coordinates so surgeons can follow the planned path.
The hospital said it uses robot-assisted brain surgery to treat a range of brain disorders. One example is inserting stereoelectroencephalography electrodes in patients with drug-resistant epilepsy to pinpoint where seizures begin. Placing one electrode typically takes 15 to 20 minutes by hand, but can be reduced to 4 to 5 minutes with a robot, the hospital said. With an average of about 15 electrodes inserted per patient, the time savings can be substantial.
Severance Hospital said its 500 cases include 327 brain tissue biopsies, 107 stereoelectroencephalography electrode insertions, 57 deep brain stimulation procedures and nine catheter insertions.
* This article has been translated by AI.
Copyright ⓒ Aju Press All rights reserved.
